Academic Success Center
Resources
Find helpful resources below to support your learning and academic success.
Student help desk: How do I contact customer support?
Please reach out to us via our Contact page or call us at 800-411-1970. We’re available Monday-Friday from 8 a.m. to midnight, and Saturday-Sunday from 8 a.m. to 10 p.m. Eastern Time.
How-to videos:
Find answers to the most common questions asked by students by discipline, from the ASC tutors.
Academic support
- Log in to your Canvas class
- Click Assignments in the menu
- Click the correct assignment name
- Click Submit Assignment
- Choose File Upload
- Select your file (preferably PDF)
- Click Submit Assignment
No, you do not have to buy a code or need a course ID.
Steps to access:
- Go to Canvas
- Click on Student MyLab Links
- Click on Course Material
- Sign in using your Goodwin email
- Go to Canvas
- Click on Modules
- Click on StudentsMyLab Links
- Click on Pearson course materials
- Open MyLab & mastering
- On the left tabs, click Multimedia Library
- Select the desired unit and content type
To schedule an appointment, visit the Meet the Tutors page and use Calendly or email the tutors directly.
For workshops, visit the Workshops page.
Study and memorization strategies
Start by grouping related terms into categories (e.g., “cell structures,” “types of pathogens”) and creating connections between them. Use flashcards, visuals, and active recall rather than rereading notes; repetition is key!
Begin by identifying the major topics or learning objectives for each lecture and focus on understanding the big picture first. Then, review a few slides at a time, summarizing key ideas in your own words or making diagrams — this helps you learn efficiently without getting overwhelmed.
Biology
DNA is a double-helix molecule made of four bases — A, T, C, and G — that pair in a specific way to encode genetic information. The sequence of these bases forms genes, which provide the instructions for building proteins and determining inherited traits.
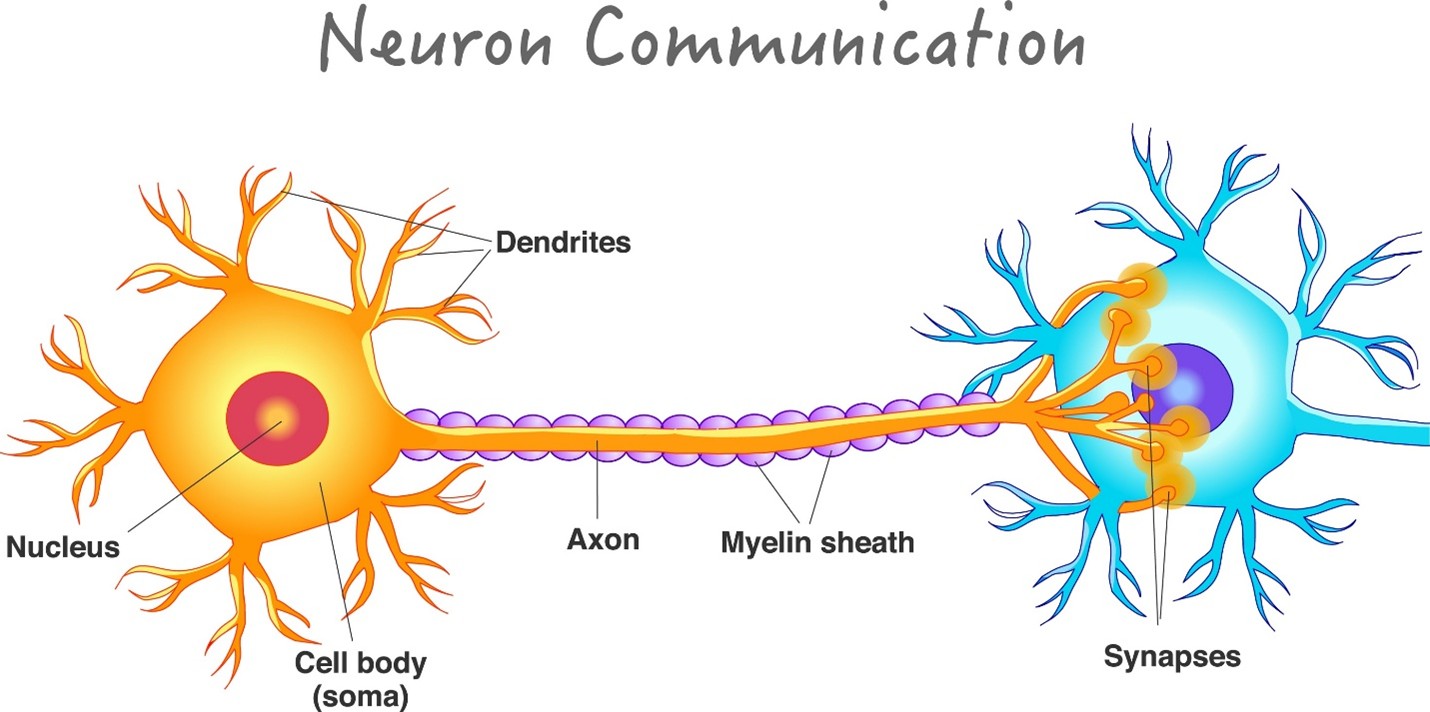
Neurons use electrical impulses called action potentials to transmit information along their axons. When the impulse reaches the end of the neuron, it triggers the release of neurotransmitters, which carry the signal across the synapse to the next cell.
Diseases can spread through direct contact (touching/bodily fluids), indirect contact (contaminated surfaces), airborne particles, or vectors such as mosquitoes and ticks.
Prokaryotic (e.g., bacteria) lack a nucleus and membrane-bound organelles.
Eukaryotic (e.g., animal, plant) have a nucleus and organelles (mitochondria, ER).
Stores genetic information (controls growth, development, and reproduction). Provides instructions for making proteins.
They are opposite processes.
Photosynthesis (plants) makes glucose and oxygen (stores energy).
Cellular Respiration (animals/plants) uses glucose and oxygen to make ATP (releases energy).
They are biological catalysts that speed up chemical reactions (e.g., digestion, metabolism) without being used up.
Chemistry
A mole is a fixed number of atoms, ions, or molecules. Just as a dozen is always 12, whether it be eggs or pumpkins, so a mole is always the same number: 6.02x1023, regardless of whether it is water molecules, iron atoms or pumpkins. It is a huge number - six followed by 23 zeros, but it is fixed and is critical for calculations in chemistry.
Valence electrons (outer-shell electrons) are the only electrons that participate in chemical reactions. They directly determine how elements react with each other and form bonds.
All metals form positive ions by losing electrons. For Group 1A, 2A, and 3A elements, the charge is equal to the number of valence electrons, which is the same as the group number. For metals that can form more than one ion, the charge is denoted by a Roman numeral. E.g., Chromium (III) means the charge on the chromium ion is plus 3 (Cr3+); Copper (I) means the copper ion has a charge of plus 1 (Cu+).
Moles (mols): This is a unit for the amount of substance. It is calculated by dividing the mass of a compound you have by its molar mass.
Molarity (M): This is a unit of concentration. It is expressed as the number of moles of a substance per liter of solution. Molarity only applies to solutions.
Math
First, set the problem up by placing what is known on the left side of the equation and what is unknown on the right side of the equation.
=
1 kilogram
2.2 pounds
? kilograms
53 pounds
Cross multiply the numbers.
1 x 53 = 53
Divide the answer found in the previous step by the remaining number.
53 ÷ 2.2 = 24.1
Therefore, 53 pounds ≈ 24.1 kilograms.
In order to solve the given problem, first calculate the milliliter per hour infusion rate. To calculate the milliliter per hour infusion rate, use dimensional analysis or the formula given below where V is the volume in milliliters, t is the time in hours, and F is the flow rate in milliliters per hour to the nearest whole number.
= F
V
t
Here the problem is solved by using the formula = F. Identify the values of V and t for the given problem.
V
t
V = 750 milliliters and t = 3 hours
Substitute 750 milliliters for V and 3 hours for t into the formula. Solve for the infusion rate.
V
t
= F
750 milliliters
3 hours
= F
250 milliliters per hour = F
Thus, the rate of infusion is 250 milliliters per hour. Use this rate to calculate the total volume to be infused in forty-five minutes.
Convert forty-five minutes into hour.
45 minutes = or hour
45
60
0.75
To calculate how much fluid will be infused, use dimensional analysis or the formula given below, where V is volume in milliliters (mL), t is time in hours, and F is flow rate in milliliters per hour.
V = t x F
Substitute hour for time t and milliliters per hour for flow rate F into the formula. Solve for the total volume, rounding to the nearest milliliter.
0.75 250
V = t x F
= 0.75 hour x 250 milliliters per hour
= 188 milliliters
So, the patient should receive 188 mL of dextrose water every forty-five minutes.
Writing
From the Hoffman Family Library page:
- Start with Single Search
- Check the box for “full text.”
- Filter “peer reviewed” from the list of search results
An article summary challenges the writer to locate the author’ts main point (who, what, where, and the theme or argument), usually written in one or several paragraphs.
An annotated bibliography covers 3-5 reference sources and asks for a 2-3 sentence paragraph (the annotation) on each source, along with the citation.
Consult the APA website for details.
See also: Starting an Annotated Bibliography in Academic Writer
Consider your thesis to be the main point you wish to convey. Your viewpoint should be narrowed and focused. A general topic (“Sickle Cell Anemia”) is too broad; limit the topic (e.g., “certain drug therapies for Sickle Cell”) to provide maximum coherence.
Anything that isn’t your own thoughts or ideas should be cited. Citations support your commentary or personal input in your paper by providing evidence from reliable sources.
Prior to writing the paper, create an outline. An outline serves as a blueprint for your paper, keeping you focused on the main argument, key topics, and subtopics.
For more on outlines, see Types of Outlines and Samples (Purdue OWL)
The thesis is the foundation of your paper — the main claim that all other parts support. It is one arguable statement that is neither too narrow nor too broad, and it is included in the introduction.
For more on thesis statements, see Tips and Examples for Writing Thesis Statements (Purdue Owl)
A professional tone:
- Excludes colloquial or conversational verbiage, idioms, and clichés.
- Requires careful word choice and avoidance of vague descriptors.
- Is achieved by appropriately considering your audience.
For more on tone, see Tone in Business Writing (Purdue Owl)
Work in small chunks of time, breaking larger projects into smaller parts. Assess what you want to accomplish, use rewards for completing work, and time your breaks (don’tt exceed 10 minutes).
Learning looks different for everyone — and that’s a good thing. Tips for Every Learner delivers quick, research-backed strategies rooted in the CAST Universal Design for Learning Guidelines™ (UDL; 2024). This framework recognizes learner variability and empowers you to build the skills that matter: using feedback effectively, staying motivated through challenges, and discovering what works for you.
Choose the format that works best for you:
- Tip sheets - One-page, printable guides with practical strategies you can use right away. Each tip sheet focuses on a specific skill and gives you concrete actions to try.
- Podcast - Prefer to listen? Each episode covers the same content in audio format, perfect for learning on the go. Listen to episodes at Tips for Every Learner.
Topics
Start with any topic that speaks to you and select the link. The skills you build here will serve you throughout your education and career.
- Integrity: Character, Values, and Professionalism
- Bringing What You Know to Your Learning
- Finding Your Place: Building Belonging and Community
- The Power of Setting Meaningful Goals
- Making the Most of Instructor Feedback
- Give Your Brain More Ways to Learn
- Making Meaningful Choices in Your Learning
- Making Learning Relevant
- Using AI as a Learning Partner
- Taking Ownership of Your Learning
Note: Podcasts use AI-generated narration. Content developed with AI, based on the CAST UDL Guidelines™, scholarly sources, and web resources.
